Describe Why Hydrogen Bonds Form Between Water Molecules.
A covalent bond in chemistry is a chemical link between two atoms or ions in which the electron pairs are shared between them. Pure water and sodium chloride crystals are both poor conductors of electricity by themselves.
How does this phenomenon demonstrate the theory of ionic bonding.

. However if sodium chloride crystals are dissolved in pure water the solution becomes a good conductor of electricity. A covalent bond may also be termed a molecular bond. It shows that the attractive forces between ions are not as strong as those.
Covalent bonds form between two nonmetal atoms with identical or relatively close electronegativity values. This type of bond may also be found in other chemical species such.

Hydrogen Bonds In Water Article Khan Academy

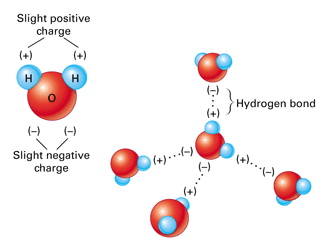
The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

Hydrogen Bonding Chemistry For Non Majors

2 1j Hydrogen Bonding And Van Der Waals Forces Biology Libretexts
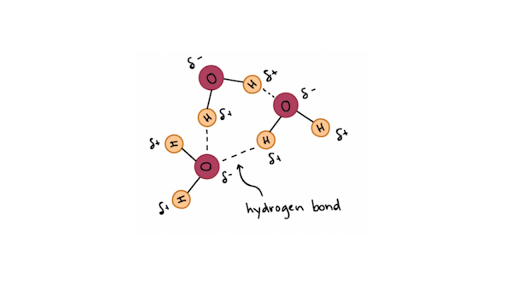
Hydrogen Bonds In Water Article Khan Academy

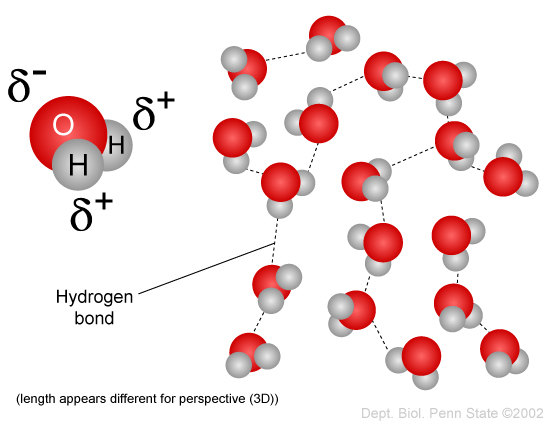
File 210 Hydrogen Bonds Between Water Molecules 01 Jpg Wikimedia Commons

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

No comments for "Describe Why Hydrogen Bonds Form Between Water Molecules."
Post a Comment